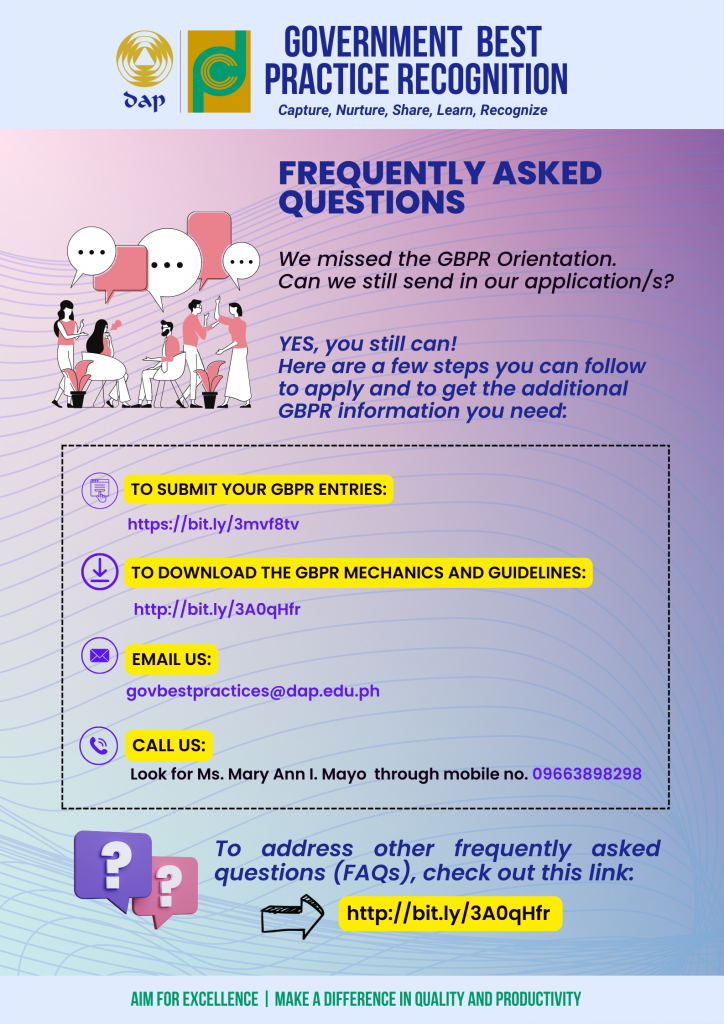
The Food and Drug Administration (FDA)’s formulation, implementation, and assessment functions are being strengthened to come up with effective and efficient regulations. Thirty-three key officers and technical staff from the different offices of the FDA attended the Basic Training Course on Regulatory Impact Assessment (RIA) and the Course on Regulatory Compliance Cost Assessment (RCCA): Cost Modelling and Streamlining last 9-10 & 13-14 and 16-17 & 20-21 December 2021, respectively.
These offices are the: Field Regulatory Operations Office (FROO), Center for Cosmetics and Household/Urban Hazardous Substances Regulation and Research (CCHUHSRR), Center for Device Regulation, Radiation Health and Research (CDRRHR), Common Services Laboratory (CSL), Center for Drug Regulation and Research (CDRR), Policy and Planning Service (PPS), and the Center for Food Regulation and Research (CFRR).
On the Basic Course on RIA, participants learned the importance of regulatory reform, RIA, its steps, and its application through the lectures and workshops. The Course on RCCA, on the other hand, featured the introduction and estimation of various regulatory compliance costs and how they may be reduced to eventually streamline FDA’s processes. Participants were exposed to the types of regulatory compliance costs, the conduct of process streamlining and were given the opportunity to revisit the cost of their own processes via a Web-based Regulatory Cost Model Calculator to help them identify where undue regulatory burden can be reduced.
The courses were conducted by the Development Academy of the Philippines (DAP), through its Modernizing Government Regulations (MGR) Program. For customized training courses on RIA, kindly email mgr@dap.edu.ph
XXX